
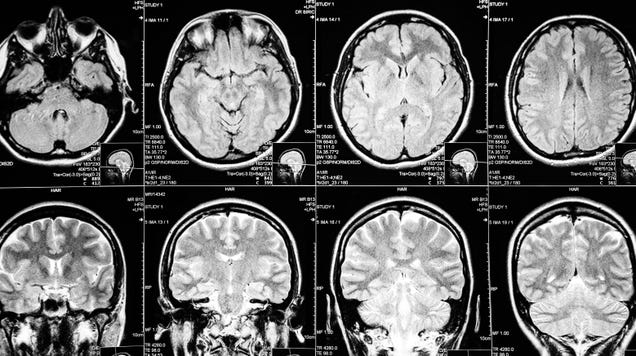
The Food and Drug Administration on Friday granted the conditional approval of a new treatment for people with early Alzheimer’s disease: the antibody-based drug lecanemab, jointly developed by the pharmaceutical companies Biogen and Eisai. In a large trial, lecanemab appeared to slow down patients’ cognitive decline…








